Tirz. 10 mg
$140.00
In stock
| Quantity | Quantity | Price per Vial |
|---|---|---|
| Quantity Based Discount | 2 - 4 | 5% $133.00 |
| Quantity Based Discount | 5 - 9 | 10% $126.00 |
| Quantity Based Discount | 10 - 19 | 15% $119.00 |
| Quantity Based Discount | 20 + | 25% $105.00 |
What is Tirzepatide?
Tirzepatide is a synthetic peptide developed for research purposes, classified as a dual receptor agonist targeting both the glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptors.
This unique mechanism sets it apart from earlier GLP-1–only compounds by potentially engaging multiple hormonal pathways involved in energy balance, insulin signaling, and appetite regulation.
In preclinical studies, this peptide has drawn interest for its ability to mimic and enhance the effects of two naturally occurring incretin hormones—GLP-1 and GIP. These hormones are released by the gut in response to nutrient intake and play key roles in stimulating insulin secretion, reducing glucagon levels, delaying gastric emptying, and influencing appetite.
Tirzepatide is provided as a high-purity research peptide in lyophilized powder form, suitable for reconstitution under sterile laboratory conditions.
|
Note: Tirzepatide is not approved for human use or therapeutic applications. This compound is intended strictly for in vitro or preclinical research and must be handled by qualified professionals in accordance with relevant guidelines and regulations. |
Mechanism of Action (Based on Research)
Tirzepatide functions as a dual agonist of the GLP-1 (glucagon-like peptide-1) and GIP (glucose-dependent insulinotropic polypeptide) receptors, which are key components of the incretin system.
GLP-1 and GIP are naturally occuring hormones secreted by the gut in response to food intake, where they play a major role in regulating insulin release, glucose balance, and appetite signals.
By activating both receptors at once, Tirzepatide appears to engage complementary pathways involved in metabolic homeostasis, making it a promising peptide for studies focused on energy regulation.
Research on GLP-1 and GIP Activation
In laboratory settings, GLP-1 receptor activation has been shown to enhance insulin secretion, reduce glucagon levels, and slow gastric emptying, all of which contribute to stabilizing blood glucose levels after meals.
GIP receptor activation, while historically less emphasized, also plays a role in insulin sensitivity and fat metabolism. Interestingly, when both receptors are stimulated together—as with Tirzepatide—the result may be greater than the sum of its parts, offering improved glucose control and appetite regulation in animal models compared to GLP-1 agonism alone.
In other words, the peptide may help the body respond more efficiently to food, improving how it handles sugar and energy storage.
Research has also shown that this dual activity impacts the central nervous system, potentially altering appetite-related signals in the brain, as well as peripheral systems involved in lipid metabolism and energy expenditure.
|
Summary: These mechanisms are still under active investigation, but the early data suggest that Tirzepatide’s multi-pathway engagement could make it an important compound in the broader study of obesity, insulin resistance, and metabolic disorders. |
Research Applications
Tirzepatide has drawn considerable attention in preclinical and observational research for its potential effects on metabolic function, particularly in studies focused on obesity, insulin regulation, and energy expenditure.
While tirzepatide peptide is not approved for medical use, findings from animal models and in vitro studies suggest this dual-agonist peptide may offer advantages over single-pathway compounds due to its ability to influence both GLP-1 and GIP receptor signaling.
Obesity Management
In animal models simulating diet-induced obesity, Tirzepatide has been associated with significant reductions in body weight.
Researchers attribute this to a combination of reduced food intake mainly through delayed gastric emptying, appetite suppression, and increased energy expenditure, likely influenced by central and peripheral hormone signaling.
Unlike GLP-1–only agonists, Tirzepatide has been noted in some research models and anecdotal reports to preserve or improve energy levels, potentially reducing fatigue that may be observed with single-pathway incretin mimetics during long-term study periods.
|
Note: Anecdotal feedback from research participants and some observational studies have also reported episodes of reduced energy or mild fatigue during Tirzepatide administration. These differences could reflect variability in individual response or dosing protocols within experimental designs. |
Metabolic Syndrome Research
Tirzepatide has also been studied in the context of metabolic syndrome, a cluster of conditions including elevated blood pressure, abnormal lipid profiles, insulin resistance, and excess body fat.
In preclinical trials, the peptide has been shown to influence multiple aspects of this syndrome by promoting insulin sensitivity, improving glucose utilization, and reducing markers of systemic inflammation. The dual-receptor approach may allow for more comprehensive modeling of metabolic corrections than GLP-1 agonism alone.
Diabetes Model Research
Several rodent studies have explored Tirzepatide in type 2 diabetes models, where it demonstrated improvements in glucose tolerance, HbA1c levels, and pancreatic beta-cell function.
GIP receptor activation appears to enhance insulin release in a glucose-dependent manner, complementing GLP-1’s effects and potentially reducing the risk of hypoglycemia in certain models. Additionally, early-stage research suggests possible protective effects on pancreatic islet structure and function under chronic metabolic stress.
These findings are based on controlled laboratory research only. Tirzepatide is not intended for human use or therapeutic applications.
Tirzepatide Peptide Characteristics
- Molecular Formula: C225H348N48O68
- CAS Number: 2023788-19-2
- Amino Acid Sequence:
- 39 amino acid linear synthetic peptide conjugated to a C20 fatty diacid moiety
- Note: The full sequence includes proprietary modifications to enhance half-life and receptor activity.
- Synonyms:
- LY3298176
- Dual GIP/GLP-1 receptor agonist
- Tirzepatide acetate (research form)
- Molar Mass: 4813.5 Da (may vary slightly depending on salt form)
- Storage Recommendations:
- Store lyophilized peptide between 36°F and 46°F (2°C and 8°C) in a dry, sealed container. If storing long-term (months to 1-2 years), store below 0°C.
- Do not freeze the compounded form of tirzepatide to avoid damaging it
- Reconstituted solutions should be stored at 2–8°C and used within a limited timeframe, depending on solvent and laboratory conditions.
- Avoid repeated freeze-thaw cycles to maintain peptide integrity.
Remember, tirzepatide is for research use only. Not for human use or therapeutic purposes.
Tirzepatide vs Semaglutide vs Retatrutide (Comparison)
|
Feature |
Semaglutide |
Tirzepatide |
Retatrutide |
|
Type |
Single agonist (GLP-1) |
Dual agonist (GLP-1, GIP) |
Triple agonist (GLP-1, GIP, Glucagon) |
|
Targets |
Appetite, blood sugar |
Appetite, blood sugar |
Appetite, blood sugar, energy expenditure |
|
Mechanism Complexity |
Simplest (single action) |
Moderate (dual action) |
Most complex (triple action) |
|
Research Stage |
FDA Approved (Ozempic®/Wegovy®) |
FDA Approved (Mounjaro®) |
Preclinical/Early Clinical Trials |
|
Weight Loss Potential (Research) |
High (15–20% at 68 wks) |
Very high (~20% over 72 weeks) |
Potentially highest (24% at 48 wks in early trials) |
|
Impact on Glucose Regulation |
Strong |
Strong |
Strong (early studies) |
|
Additional Effects |
Cardiovascular benefits |
Insulin sensitivity improvements |
Increased energy expenditure (animal models) |
|
Dosing Frequency |
Weekly |
Weekly |
TBD (under investigation) |
|
Approval Status |
Approved medication |
Approved medication |
Research use only |
|
Disclaimer |
Approved for medical use |
Approved for medical use |
Research use only, not for human consumption |
Safety and Side Effects in Studies
Current findings on Tirzepatide are largely based on preclinical and early clinical research, with many insights drawn from animal models and laboratory-controlled studies. In these settings, Tirzepatide has demonstrated a generally favorable safety profile, particularly at dosages typically used for research purposes.
Across various non-clinical models, adverse effects have been limited and not widely reported, with most subjects tolerating the compound well.
That said, it’s important to note that responses can vary depending on species, dosage, and experimental conditions. The absence of severe side effects in research contexts does not imply safety in humans.
Tirzepatide is provided for scientific research only. It is not intended for human consumption, diagnostic use, or therapeutic treatment.
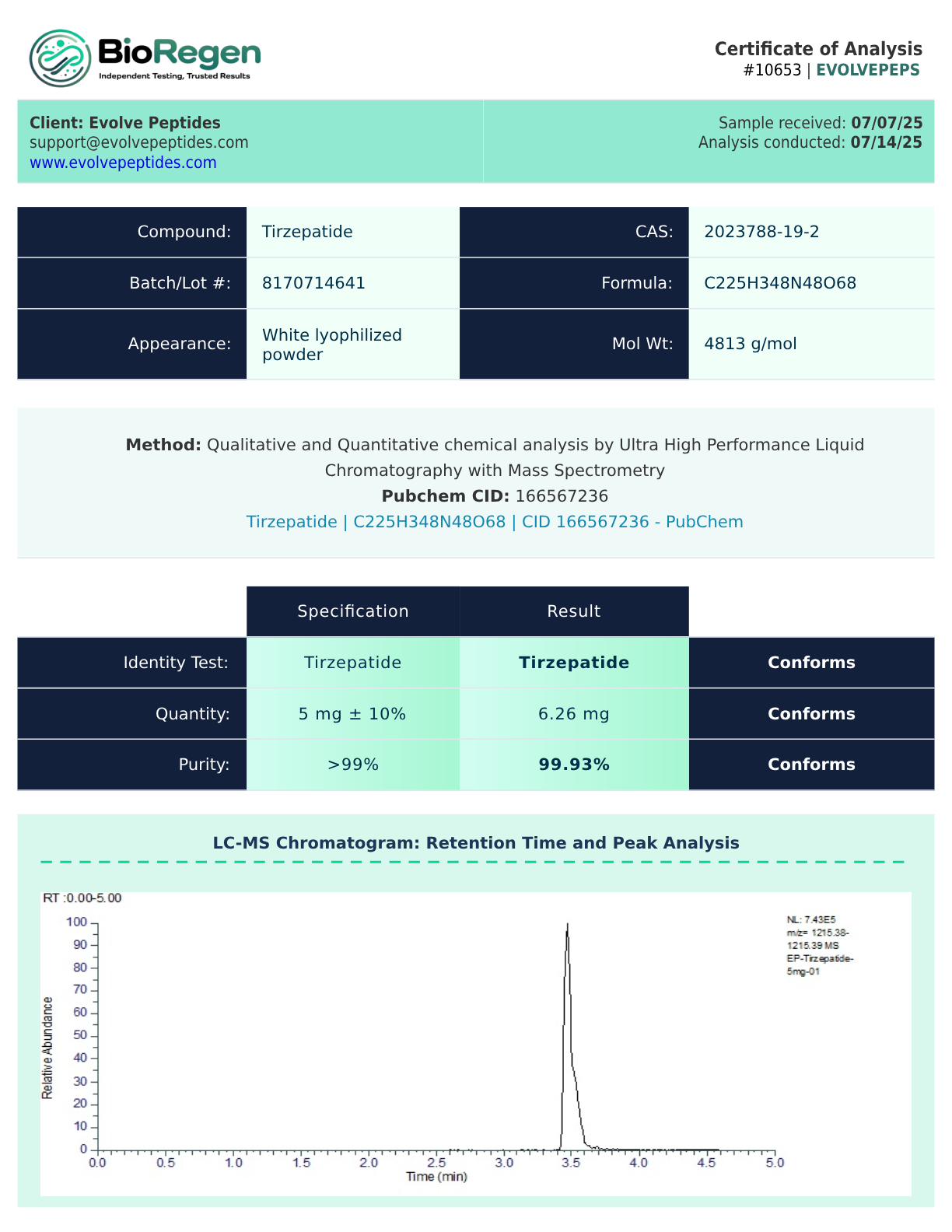
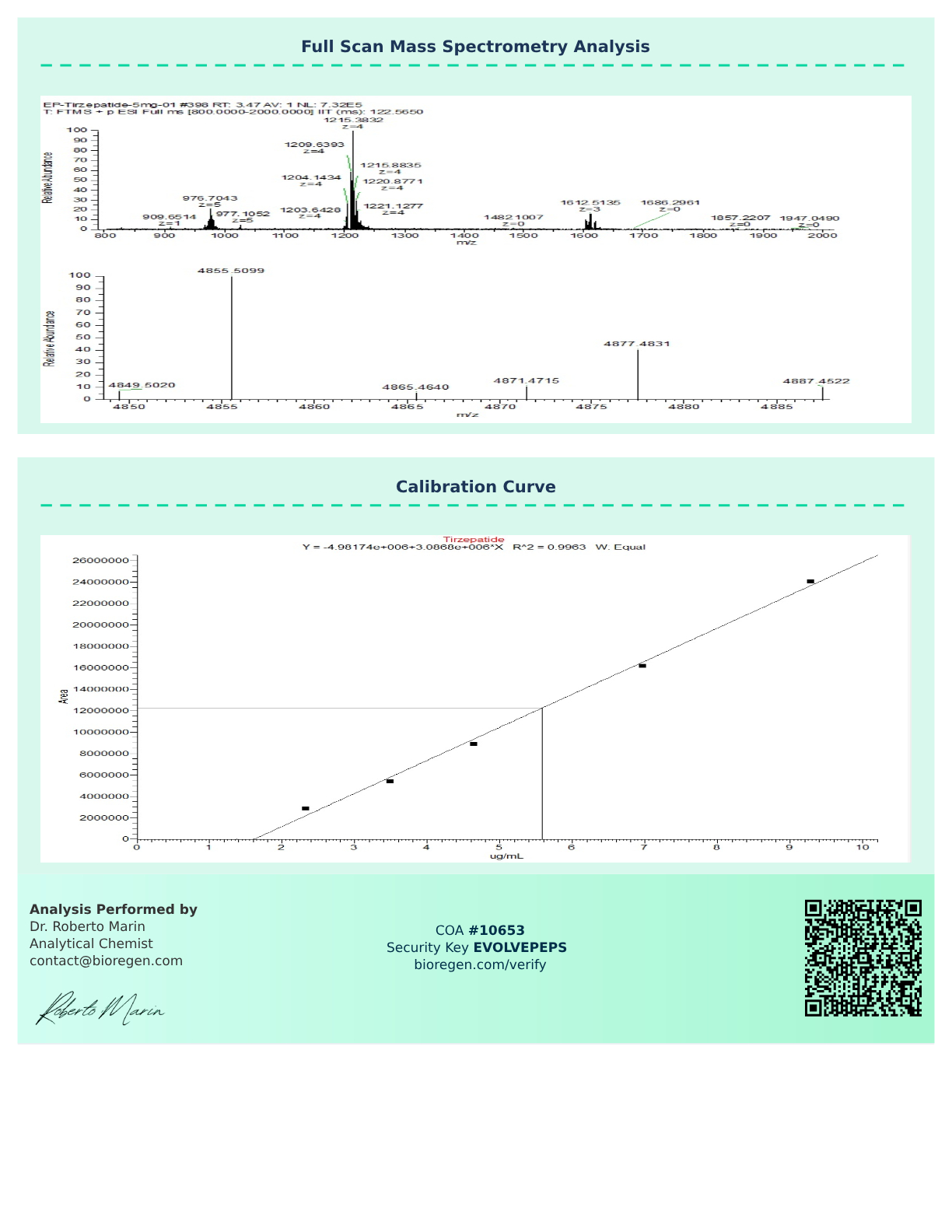
Certificate of Analysis (COA)
Each vial of Tirzepatide peptide from Evolve Peptides is supported by a Certificate of Analysis (COA) to verify identity, purity, and molecular composition. Every batch undergoes independent third-party testing to confirm consistent quality, with purity levels that typically exceed 99.6%.
Researchers can request a COA with their order by directly contacting us, and we’ll provide one so you can conduct your research with confidence and data reliability.
Tirzepatide FAQs
What is Tirzepatide used for in research?
Tirzepatide is studied for its dual action on GIP and GLP-1 receptors, which are involved in glucose metabolism, appetite regulation, and energy balance. Researchers explore its potential role in metabolic function, insulin signaling, and weight regulation under controlled lab conditions.
Is Tirzepatide the same as GLP-1 agonists like Semaglutide?
Not exactly. While both target GLP-1 receptors, Tirzepatide also activates GIP receptors, making it a dual agonist. This broader mechanism is believed to enhance its effects on insulin sensitivity and appetite signaling in research models.
What’s the difference between Tirzepatide and Retatrutide?
Tirzepatide is a GIP/GLP-1 dual agonist, while Retatrutide is a triple agonist that also includes glucagon receptor activation. This means Retatrutide may influence energy expenditure and fat metabolism in ways Tirzepatide doesn’t, according to early studies.
How is Tirzepatide stored for research?
Lyophilized Tirzepatide should be stored in a cool, dry place, typically -20°C for long-term storage. Once reconstituted, it should be used promptly and stored under sterile, refrigerated conditions, as per lab protocol.
Scientific References
- Meloni, A. R., DeYoung, M. B., Lowe, C., & Parkes, D. G. (2013). GLP-1 receptor activated insulin secretion from pancreatic β-cells: mechanism and glucose dependence. Diabetes, Obesity and Metabolism, 15(1), 15–27.
https://doi.org/10.1111/j.1463-1326.2012.01663.x - Paula-Peace James-Okoro, Jo Edward Lewis, Fiona Mary Gribble, Frank Reimann. Frontiers in Endocrinology. (2025). The role of GIPR in food intake control. Frontiers in Endocrinology. Volume 16 – 2025
https://doi.org/10.3389/fendo.2025.1532076 https://www.frontiersin.org/articles/10.3389/fendo.2025.1532076/full - Baggio, L. L., & Drucker, D. J. (2014). Glucagon-like peptide-1 receptors in the brain: controlling food intake and body weight. The Journal of Clinical Investigation, 124(10), 4223–4226.
https://doi.org/10.1172/JCI78371
https://www.jci.org/articles/view/78371 - Ailin Zhang, Qinhui Liu, Yimin Xiong, Jiahui Li, Ying Xu, Haiying Song, Xiandan Jing, Haixia Xu, Na Yang, Yanping Li, Li Mo, Qin Tang, Jinhan He (2025). Tirzepatide reduces body weight by increasing fat utilization via the central nervous system-adipose tissue axis in male mice. Diabetes Obes Metab. 2025 May;27(5):2844-2856. doi: 10.1111/dom.16294. Epub 2025 Feb 25.
https://pubmed.ncbi.nlm.nih.gov/40000395/ - Jalleh, R. J., Plummer, M. P., Marathe, C. S., Umapathysivam, M. M., Quast, D. R., Rayner, C. K., Jones, K. L., Wu, T., Horowitz, M., & Nauck, M. A. Journal of Clinical Endocrinology and Metabolism. (2025). Clinical Consequences of Delayed Gastric Emptying With GLP-1 Receptor Agonists and Tirzepatide. The Journal of Clinical Endocrinology & Metabolism, Volume 110, Issue 1, January 2025, Pages 1–15,
https://doi.org/10.1210/clinem/dgae719
https://academic.oup.com/jcem/article/110/1/1/7824836 - [PMC Article]. (2025). Tirzepatide for MAFLD. Clinical Consequences of Delayed Gastric Emptying With GLP-1 Receptor Agonists and Tirzepatide, Volume 110 Issue 1,
https://pmc.ncbi.nlm.nih.gov/articles/PMC10923294/ - De Block, C., Bailey, C., Wysham, C., Hemmingway, A., Allen, S. E., & Peleshok, J. PMC Article. (2025). Tirzepatide for the treatment of adults with type 2 diabetes: An endocrine perspective. Diabetes Obes Metab. 2023 Jan;25(1):3-17. doi: 10.1111/dom.14831. Epub 2022 Aug 31.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10087310/ - UCLA Health. (2025). Semaglutide weight loss: What you need to know.
https://www.uclahealth.org/news/article/semaglutide-weight-loss-what-you-need-know - Ghusn, W., De la Rosa, A., Sacoto, D., Cifuentes, L., Campos, A., Feris, F., Hurtado, M. D., & Acosta, A. [PMC Article]. (2025). Weight Loss Outcomes Associated With Semaglutide Treatment for Patients With Overweight or Obesity. National Library of Medicine. Volume 5(9).
https://pmc.ncbi.nlm.nih.gov/articles/PMC9486455/ - Emily Harris. JAMA Network. (2025). Triple-Hormone Combination Retatrutide Induces 24% Body Weight Loss. JAMA. 2023;330(4):306. doi:10.1001/jama.2023.12055.
https://jamanetwork.com/journals/jama/article-abstract/2807151
Contents: 5 mg lyophilized (freeze-dried) powder provided in a 3 ml vial, sealed and sterile. Purity exceeds 99%, guaranteed.
Notes: Requires reconstitution with bacteriostatic water. (Sold Here: BAC Water.)
Orders placed before 1 PM EST ship same day.
We use USPS for most of our deliveries. You can expect your parcel within 2-5 business days from when it leaves our warehouse.
Why Choose Us?
Potency & Purity Guaranteed
Our peptides are custom-manufactured to exact specifications. Strictly no compromises.
Rigorously Third-Party Tested
Every product page includes independent lab test results for every peptide we sell.
Dedicated End-to-End Support
We're here for you every step of the way. Our goal is your success in research.
100% Satisfaction Guaranteed
If you are not 100% satisfied with our research peptides, we'll refund everything you paid.