Reta 5 mg
$90.00
In stock
| Quantity | Quantity | Price per Vial |
|---|---|---|
| Quantity Based Discount | 2 - 4 | 5% $85.50 |
| Quantity Based Discount | 5 - 9 | 10% $81.00 |
| Quantity Based Discount | 10 - 19 | 15% $76.50 |
| Quantity Based Discount | 20 + | 25% $67.50 |
What is Retatrutide?
Retatrutide is a synthetic research peptide designed to act as a triple receptor agonist, targeting the GLP-1, GIP, and glucagon receptors. It mimics and enhances the activity of natural hormones involved in energy regulation, glucose metabolism, and appetite control.
As a lab-engineered compound, retatrutide is constructed to replicate and extend the functions of these key metabolic hormones. This peptide is notable for its stability in solution and its high solubility in aqueous buffers, which makes it highly suitable for controlled laboratory settings.
Retatrutide’s structure allows for consistent receptor binding affinity and reliable results across a range of experimental models. It is currently the focus of ongoing non-clinical and preclinical studies that aim to better understand its role in metabolic signaling.
| Note: Our retatrutide peptide for sale is intended for research use only. It is not intended for human consumption, therapeutic use, or diagnostic procedures. Researchers value retatrutide for its ability to target multiple hormonal pathways simultaneously, compared to more narrowly focused peptides such as semaglutide and tirzepatide. |
Retatrutide Mechanism of Action
Retatrutide acts as a multi-pathway agonist, binding to and activating the GLP-1, GIP, and glucagon receptors. This triple-agonist mechanism allows it to influence several interconnected hormonal systems.
Retatrutide: Appetite Regulation
By stimulating GLP-1 and GIP receptors, Retatrutide appears to signal satiety centers in the hypothalamus, potentially reducing food intake in preclinical animal models. Brain regulation of appetite is one of the more promising avenues of reducing obesity and diabetes.
GLP-1 activation is also associated with delayed gastric emptying, another contributing factor to appetite suppression and thus reduced food intake.
Retatrutide: Blood Sugar Modulation
Studies suggest that Retatrutide may improve glucose tolerance by enhancing insulin secretion and decreasing glucagon release in response to food intake. It helps the body respond more effectively to rising blood sugar levels after meals.
Since retatrutide appears to support better blood sugar balance in experimental models, its dual action could make it a valuable compound for researchers studying metabolic function and glucose regulation.
These effects mirror those observed with other incretin-based therapies but may be amplified by the peptide’s triple-agonist design.
Retatrutide: Enhanced Energy Metabolism
The inclusion of glucagon receptor activity sets Retatrutide apart. Glucagon promotes energy expenditure and lipolysis, which is the breakdown of fat for fuel.
This means Retatrutide may not only influence appetite and insulin sensitivity but also support increased basal metabolic rate and fat utilization in research models.
Unlike peptides that activate only GLP-1 or GIP receptors—such as semaglutide and tirzepatide—Retatrutide’s activation of the glucagon pathway appears to sustain energy output, even during calorie restriction.
Notably, some studies have observed that research subjects experiencing fatigue or reduced energy on semaglutide or tirzepatide seemed to maintain or even regain energy levels when switched to Retatrutide.
While the exact mechanisms are still being studied, this suggests the glucagon component may play a key role in counteracting metabolic slowdown and fatigue, making Retatrutide especially interesting in long-term metabolic research.
Research Applications (Retatrutide Benefits)
Retatrutide is currently being investigated in a variety of non-clinical and preclinical models, particularly those related to metabolic health. Its unique mechanism of action has positioned it as a compound of interest in the following research domains.
Weight Management Studies
Animal research indicates that Retatrutide may produce significant body weight reductions through mechanisms involving reduced calorie intake and increased energy expenditure.
In preclinical trials, rodents treated with Retatrutide exhibited a marked reduction in food intake, often within days of starting treatment. This is thought to result from enhanced satiety signaling in the brain, particularly in the hypothalamus, where these gut hormone pathways converge to regulate hunger.
Animal studies have also reported elevated resting energy expenditure. This effect is likely mediated by the glucagon receptor agonism component, which is known to promote thermogenesis and lipolysis—essentially encouraging the body to burn more calories, even at rest.
In one study conducted in diet-induced obese mice, Retatrutide not only led to significant reductions in body weight—often surpassing those seen with GLP-1-only therapies—but also improved metabolic parameters such as insulin sensitivity, lipid profiles, and hepatic fat content.
| Note: Importantly, weight loss continued over time rather than plateauing early, suggesting a durable metabolic effect. These outcomes are being further evaluated to better understand the peptide’s full range of metabolic interactions. |
Metabolic Function Research
Retatrutide’s influence on glucose tolerance, insulin sensitivity, and lipid metabolism makes it a useful tool for exploring the hormonal and cellular pathways tied to type 2 diabetes, insulin resistance, and non-alcoholic fatty liver disease (NAFLD).
In animal models, Retatrutide has consistently improved glucose handling, with treated subjects demonstrating enhanced glucose tolerance during oral glucose tolerance tests (OGTTs). This suggests that the drug helps the body clear glucose from the bloodstream more efficiently.
In parallel, Retatrutide also exerts favorable effects on lipid metabolism. Treated animals often exhibit reductions in circulating triglycerides and LDL cholesterol, along with lower levels of hepatic steatosis (fat accumulation in the liver).
These changes are likely due in part to glucagon receptor activation, which promotes lipid oxidation and decreases lipogenesis in the liver. The result is a decrease in ectopic fat storage—a hallmark of insulin resistance and NAFLD.
Hormonal Modulation
Retatrutide’s unique ability to interact with multiple receptor types provides a framework for studying hormonal cross-talk, especially how GLP-1, GIP, and glucagon signaling affect one another in metabolic tissues.
When engaged together, these interactions reveal complex synergistic or opposing effects across tissues like the liver, pancreas, adipose tissue, and brain.
For example, while GLP-1 suppresses appetite, glucagon may counterbalance this by increasing thermogenesis, suggesting a coordinated push toward energy balance.
This tri-agonist model allows researchers to dissect how hormonal signals converge to influence glucose homeostasis, insulin action, and fat storage, providing deeper insights than single-pathway agents.
These observations are based on animal and in vitro research models. Retatrutide is not approved for human use.
Retatrutide Peptide Characteristics
- Molecular Formula: C223H343F3N46O70
- CAS Number: 2381089-83-2
- Amino Acid Sequence: [Sequence not publicly disclosed; proprietary to research studies involving Eli Lilly & Co.]
- Synonyms: LY3437943; Triple Agonist GLP-1/GIP/Glucagon Analog
- Molar Mass: 4731.34 g/mol
- Storage Recommendations: Keep container tightly sealed in cool, well-ventilated area away from direct sunlight and sources of ignition.
- Store at −20°C for up to one year (sealed)
- Store at −80°C for up to two years (sealed)
- Can be shipped and kept at room temperature for up to two weeks.
- Avoid repeated freeze-thaw cycles to maintain structural integrity
Retatrutide vs Tirzepatide vs Semaglutide Comparison
| Feature | Semaglutide | Tirzepatide | Retatrutide |
| Type | Single agonist (GLP-1) | Dual agonist (GLP-1, GIP) | Triple agonist (GLP-1, GIP, Glucagon) |
| Primary Targets | Appetite, blood glucose | Appetite, blood glucose | Appetite, blood glucose, energy expenditure |
| Mechanism Complexity | Single pathway | Dual pathway | Most complex (multi-pathway) |
| Research/Approval Stage | FDA approved (Ozempic®, Wegovy®) | FDA approved (Mounjaro®, Zepbound®) | Not FDA-approved for human use (Phase 1/2 clinical trials) |
| Weight Loss Potential | High (15–20% at 68 wks) | Very high (~20% over 72 weeks) | Potentially highest (24% at 48 wks in early trials) |
| Glucose Control | HbA1c reduction by 1.5–1.8% in 30–56 wks | HbA1c reduction ~1.94% | HbA1c levels below 6.5% in early human & animal data |
| Additional Benefits | Cardiovascular risk reduction | Improved insulin sensitivity | Increased energy expenditure, hepatic fat reduction |
| Dosing Frequency | Weekly | Weekly | Weekly (subcutaneous) |
| Regulatory Status | Approved for medical use | Approved for medical use | Research use only |
| Disclaimer | Prescription-only medication | Prescription-only medication | Not approved for human use; investigational agent |
Safety and Side Effects in Studies
To date, most available data on Retatrutide comes from preclinical research, primarily in animal models. At research-level dosages, studies have not reported extensive adverse effects.
In rodent studies, Retatrutide has been generally well tolerated, with side effects typically limited to those commonly seen with incretin-based therapies, such as transient gastrointestinal symptoms. However, it’s critical to note that these findings do not imply safety in humans.
No human safety profile has been established beyond early-phase trials, and this compound is not approved for therapeutic use. Retatrutide is intended strictly for laboratory research and is not for human consumption.
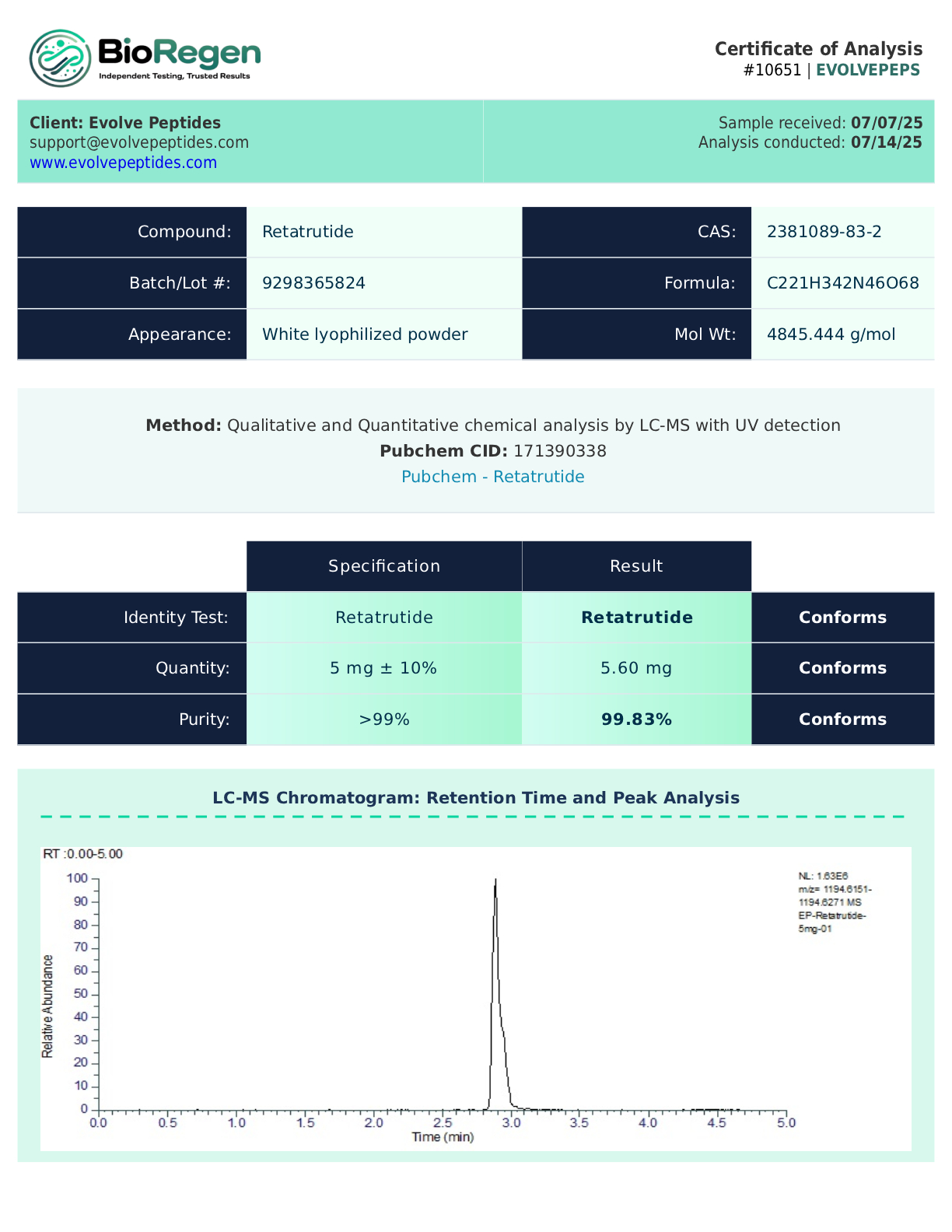
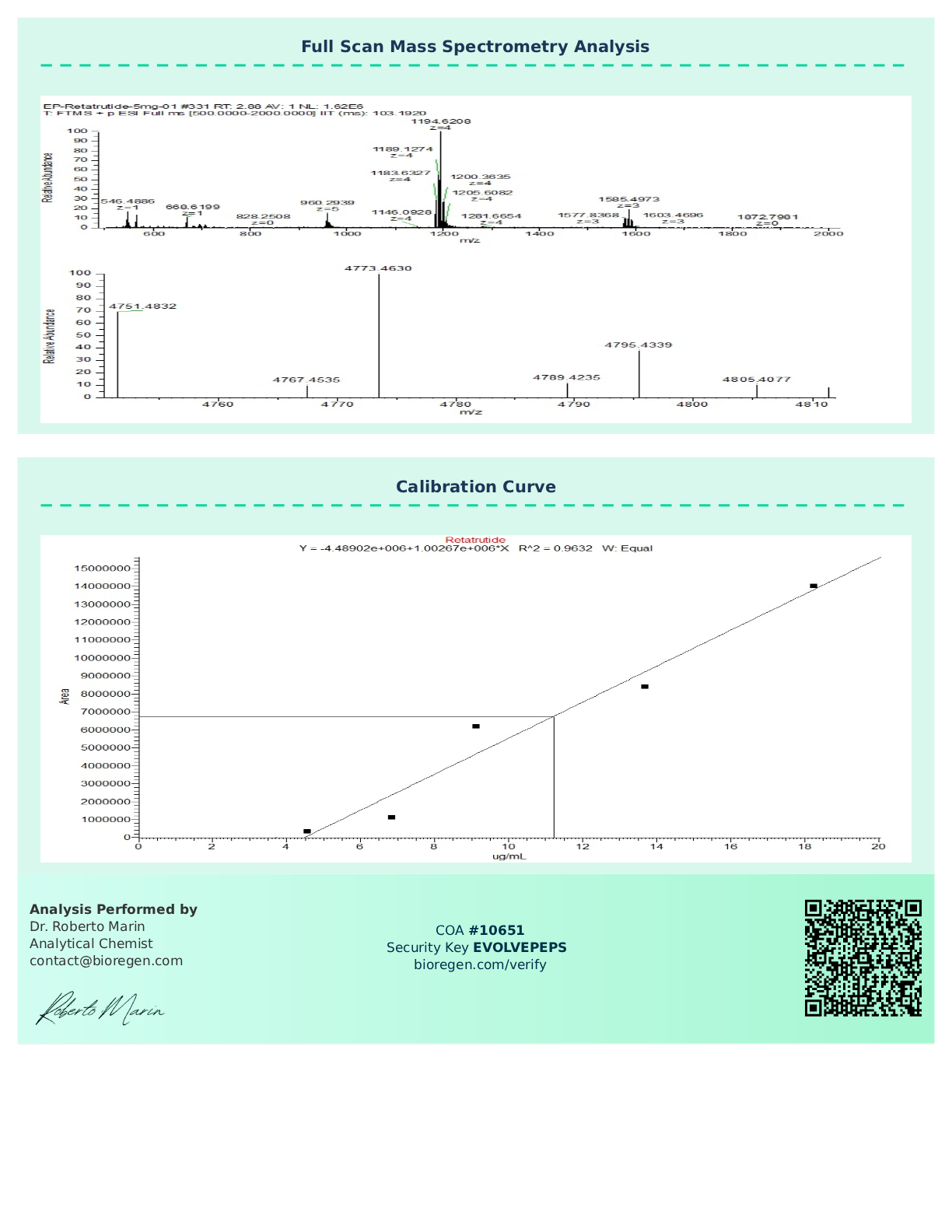
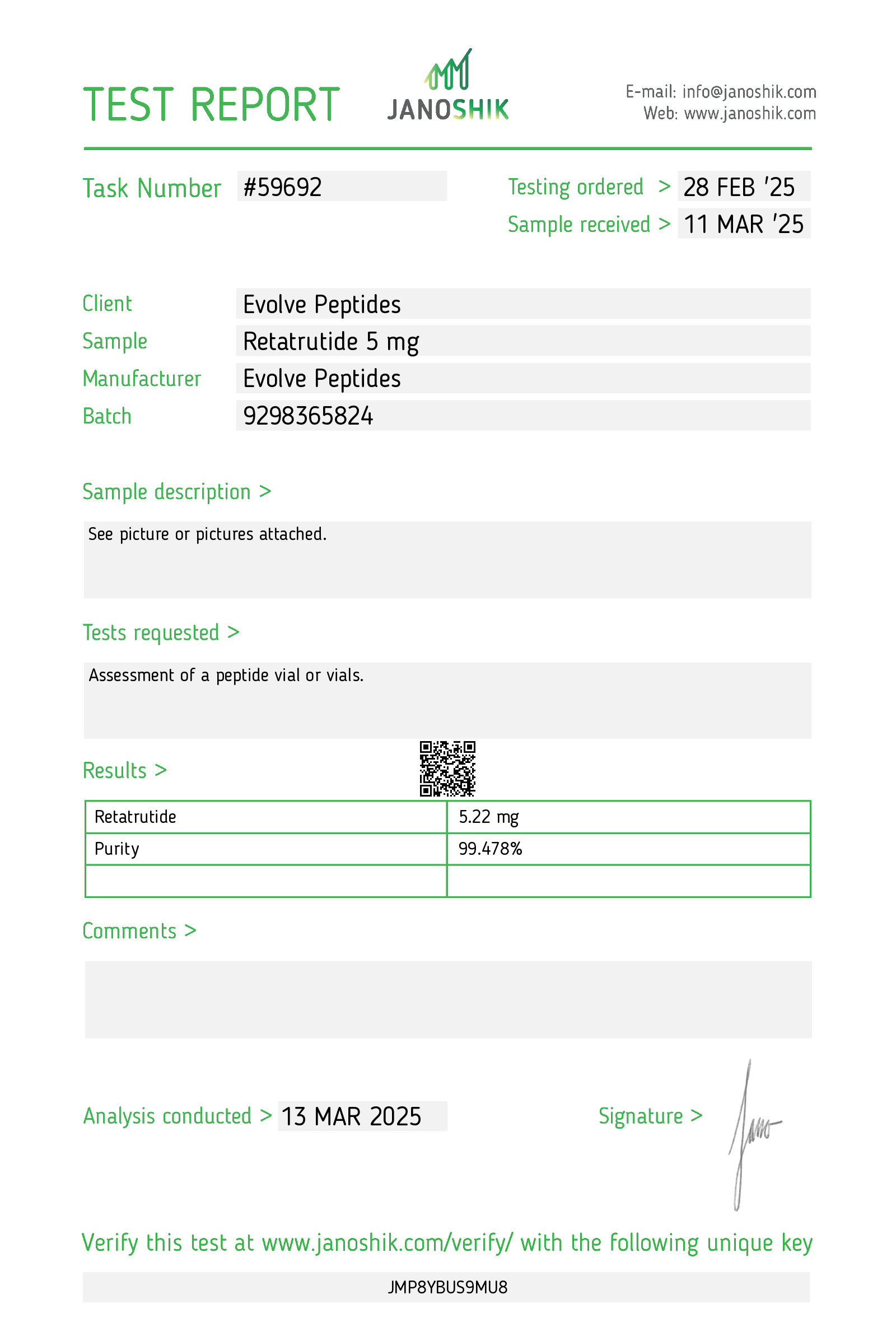
Certificate of Analysis (COA)
Each batch of Retatrutide is subjected to third-party laboratory testing to verify purity, identity, and quality. A Certificate of Analysis (COA) is available upon request to ensure transparency and reproducibility in research settings.
Legal Disclaimer
This product is intended for laboratory research purposes only and is not approved for human or veterinary use.
Not for resale, diagnostic, or therapeutic applications.
Retatrutide FAQs
Is Retatrutide approved for human use?
No. Retatrutide is currently in early-stage clinical trials and is not approved for human or veterinary use.
How does Retatrutide compare to other weight-loss drugs?
Early studies suggest Retatrutide may produce greater weight loss than GLP-1 or dual agonists like Tirzepatide, due to its triple-action mechanism. However, more research is needed.
What are the known side effects of Retatrutide?
In animal studies, side effects have been minimal and mostly gastrointestinal. Human safety data is limited, and effects are still being studied.
What is the recommended dosage schedule for Retatrutide?
There is no established dosing schedule for Retatrutide outside of clinical trials. Dosing regimens in studies are tightly controlled and vary based on the research design, but generally vary between 1–8 mg weekly, in increments of 1mg with a maximum dose of 8–12mg.
Where can I buy Retatrutide for research?
You can now buy Retatrutide in high-purity form at Evolve Peptides, a trusted supplier of research compounds. All products come with third-party testing for quality assurance, with fast and secure shipping on each order.
Scientific References
- Jastreboff, A. M., Aronne, L. J., Ahmad, N. N., Wharton, S., Connery, L., Alves, B., … & Kushner, R. F. (2023). Triple–Hormone-Receptor Agonist Retatrutide for Obesity—A Phase 2 Trial. The New England Journal of Medicine, 389(6), 514–526. https://doi.org/10.1056/NEJMoa2301972New England Journal of Medicine+2New England Journal of Medicine+2New England Journal of Medicine+2
- Ahima, R. S., & Antwi, D. A. (2008). Brain regulation of appetite and satiety. Endocrinology and Metabolism Clinics of North America, 37(4), 811–823. https://doi.org/10.1016/j.ecl.2008.08.005
- Abouelmagd, A. A., Abdelrehim, A. M., Bashir, M. N., Abdelsalam, F., Marey, A., Tanas, Y., … & Belal, M. M. (2025). Efficacy and safety of retatrutide, a novel GLP-1, GIP, and glucagon receptor agonist for obesity treatment: A systematic review and meta-analysis of randomized controlled trials. Proceedings (Baylor University. Medical Center), 38(3), 291–303. https://doi.org/10.1080/08998280.2025.2456441PubMed
- Holst, J. J., & Knop, F. K. (2023). GLP-1 and the neurobiology of eating control: Recent advances. Nature Reviews Endocrinology, 19(3), 157–171. https://doi.org/10.1038/s41574-022-00738-1PMC+1PubMed+1
- Conceição-Furber, E., Coskun, T., Sloop, K. W., & Samms, R. J. (2022). Is glucagon receptor activation the thermogenic solution for treating obesity? Frontiers in Endocrinology, 13, 868037. https://doi.org/10.3389/fendo.2022.868037
- Abouelmagd, A. A., Abdelrehim, A. M., Bashir, M. N., Abdelsalam, F., Marey, A., Tanas, Y., … & Belal, M. M. (2025). Efficacy and safety of retatrutide, a novel GLP-1, GIP, and glucagon receptor agonist for obesity treatment: A systematic review and meta-analysis of randomized controlled trials. Pharmacological Research, 190, 106789. https://doi.org/10.1016/j.phrs.2024.106789
- Sanyal, A. J., Kaplan, L. M., Frias, J. P., Brouwers, B., Wu, Q., Thomas, M. K., … & Hartman, M. L. (2024). Triple hormone receptor agonist retatrutide for metabolic dysfunction-associated steatotic liver disease: A randomized phase 2a trial. Nature Medicine, 30(7), 2037–2048. https://doi.org/10.1038/s41591-024-03018-2
- National Center for Advancing Translational Sciences. (2025). Retatrutide. Global Substance Registration System (GSRS). Retrieved from https://gsrs.ncats.nih.gov/ginas/app/ui/substances/NOP2Y096GV
- Urva, S., Coskun, T., Loh, M. T., Du, Y., Thomas, M. K., Gurbuz, S., … & Milicevic, Z. (2022). LY3437943, a novel triple GIP, GLP-1, and glucagon receptor agonist in people with type 2 diabetes: A phase 1b, multicentre, double-blind, placebo-controlled, randomised, multiple-ascending dose trial. The Lancet, 400(10366), 1869–1881. https://doi.org/10.1016/S0140-6736(22)02033-5
- MedChemExpress. (2025). Retatrutide TFA Safety Data Sheet. Retrieved from https://file.medchemexpress.com/batch_PDF/HY-P3506A/Retatrutide-TFA-SDS-MedChemExpress.pdf
- UCLA Health. (2023, January 12). Semaglutide for weight loss – what you need to know. Retrieved from https://www.uclahealth.org/news/article/semaglutide-weight-loss-what-you-need-know
- Kushner, R. F., Calanna, S., Davies, M., Dicker, D., Garvey, W. T., Goldman, B., … & Wharton, S. (2022). Semaglutide 2.4 mg for the treatment of obesity: A review of the STEP 1–5 trials. Obesity (Silver Spring), 30(6), 1040–1051. https://doi.org/10.1002/oby.23391
- Wilding, J. P. H., Batterham, R. L., Calanna, S., Davies, M., Van Gaal, L. F., Lingvay, I., … & Kushner, R. F. (2021). Once-weekly semaglutide in adults with overweight or obesity. The New England Journal of Medicine, 384(11), 989–1002. https://doi.org/10.1056/NEJMoa2032183
- Coskun, T., Urva, S., Loh, M. T., Du, Y., Thomas, M. K., Gurbuz, S., … & Haupt, A. (2021). LY3437943, a novel triple GIP, GLP-1, and glucagon receptor agonist: A phase 1b, multicentre, double-blind, placebo-controlled, randomised, multiple-ascending dose trial. Frontiers in Endocrinology, 12, 645617. https://doi.org/10.3389/fendo.2021.645617
- Zhou, Y., Zhang, Y., Wang, L., & Wang, Y. (2021). Efficacy and safety of semaglutide in non-diabetic obese adults: A systematic review and meta-analysis. Obesity Reviews, 22(12), e13313. https://doi.org/10.1111/obr.13313
- Sanyal, A. J., Kaplan, L. M., Frias, J. P., Brouwers, B., Wu, Q., Thomas, M. K., … & Hartman, M. L. (2024). Triple hormone receptor agonist retatrutide for metabolic dysfunction-associated steatotic liver disease: A randomized phase 2a trial. Nature Medicine, 30(7), 2037–2048. https://doi.org/10.1038/s41591-024-03018-2
- International Association for Physicians in Aesthetic Medicine. (2025). Retatrutide Dosage: A Guide. Retrieved from https://iapam.com/medical-weight-management-library/retatrutide-dosage-a-guide
Contents: 5 mg lyophilized (freeze-dried) powder provided in a 3 ml vial, sealed and sterile. Purity exceeds 99%, guaranteed.
Notes: Requires reconstitution with bacteriostatic water. (Sold Here: BAC Water.)
Chemical Formula: C223H343F3N46O70
PubChem SID: 474492235
CAS Number: 2381089-83-2
Molecular Weight: 4731.34 g/mol
Storage: Store at ≤8°C, sealed, away from heat, light, and moisture. The colder the better.
Purity: >99%
Orders placed before 1 PM EST ship same day.
We use USPS for most of our deliveries. You can expect your parcel within 2-5 business days from when it leaves our warehouse.
Why Choose Us?
Potency & Purity Guaranteed
Our peptides are custom-manufactured to exact specifications. Strictly no compromises.
Rigorously Third-Party Tested
Every product page includes independent lab test results for every peptide we sell.
Dedicated End-to-End Support
We're here for you every step of the way. Our goal is your success in research.
100% Satisfaction Guaranteed
If you are not 100% satisfied with our research peptides, we'll refund everything you paid.